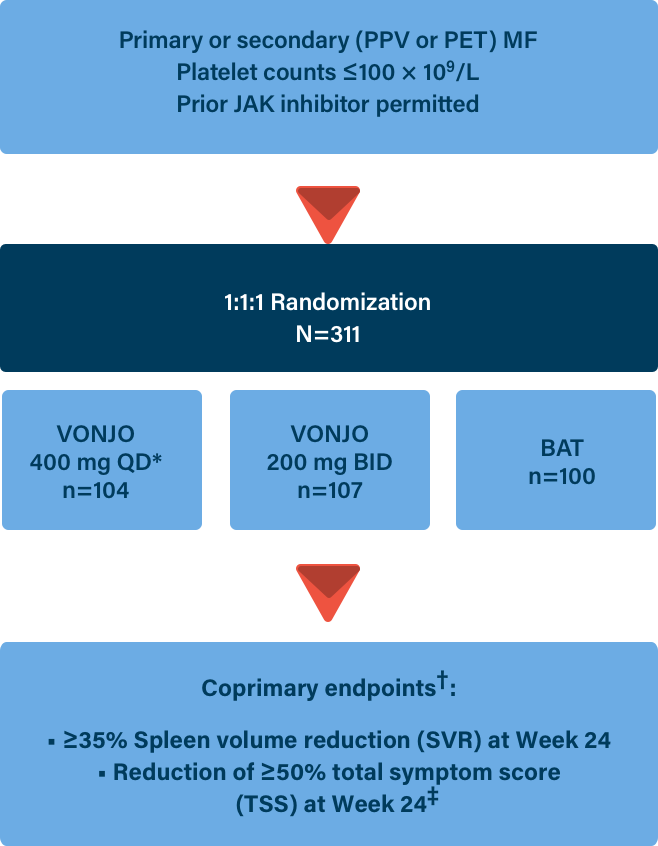
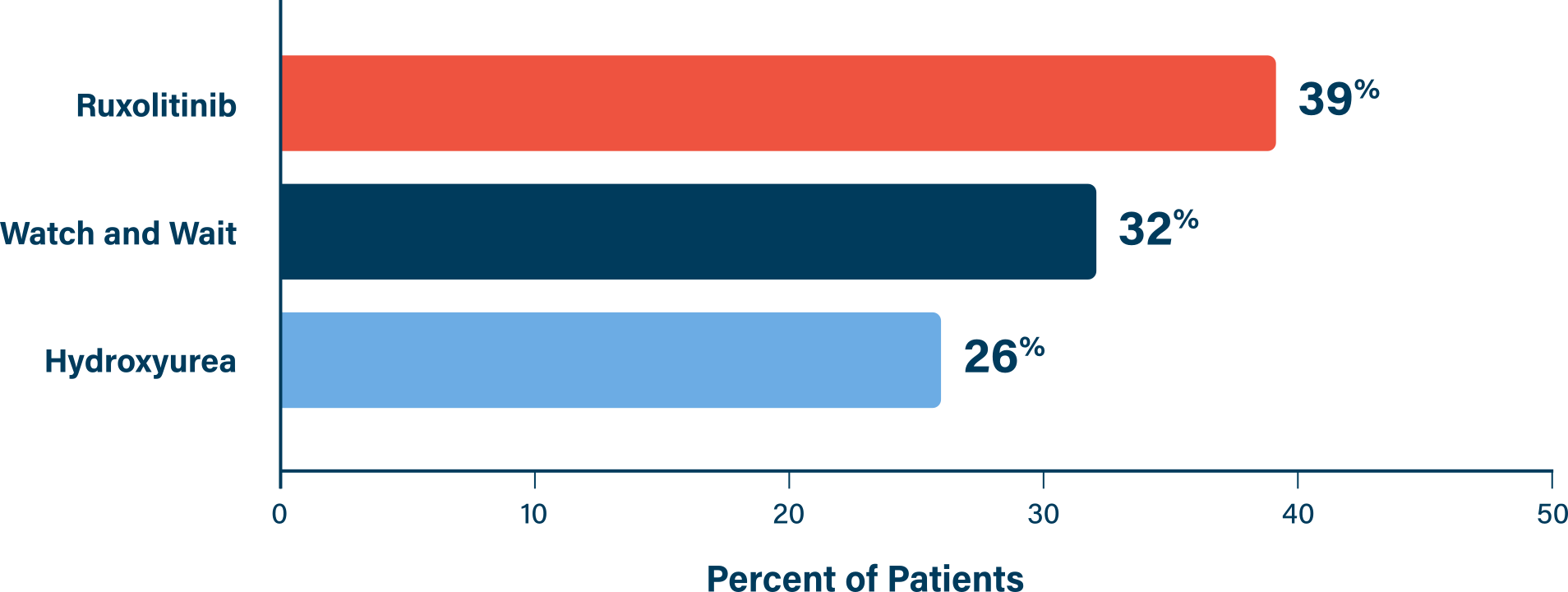
Important Safety Information
Contraindication
VONJO is contraindicated in patients concomitantly using strong CYP3A4 inhibitors or inducers.
Warnings and Precautions:
Hemorrhage: Serious (11%) and fatal (2%) hemorrhages have occurred in VONJO-treated patients with platelet counts <100 x 109/L. Serious (13%) and fatal (2%) hemorrhages have occurred in VONJO-treated patients with platelet counts <50 x 109/L. Grade ≥3 bleeding events (defined as requiring transfusion or invasive intervention) occurred in 15% of patients treated with VONJO compared to 7% of patients treated on the control arm. Due to hemorrhage, VONJO dose reductions, dose interruptions, or permanent discontinuations occurred in 3%, 3%, and 5% of patients, respectively.
Avoid use of VONJO in patients with active bleeding and hold VONJO 7 days prior to any planned surgical or invasive procedures. Assess platelet counts periodically, as clinically indicated. Manage hemorrhage using treatment interruption and medical intervention.
Diarrhea: VONJO caused diarrhea in approximately 48% of patients compared to 15% of patients treated on the control arm in clinical trials. The median time to resolution in VONJO-treated patients was 2 weeks. The incidence of reported diarrhea decreased over time with 41% of patients reporting diarrhea in the first 8 weeks of treatment, 15% in Weeks 8 through 16, and 8% in Weeks 16 through 24. Diarrhea resulted in treatment interruption in 3% of VONJO-treated patients. Serious diarrhea adverse reactions occurred in 2% of patients treated with VONJO compared to no such adverse reactions in patients in the control arm. In postmarketing reports, severe diarrhea leading to acute kidney injury and treatment discontinuation has been reported with VONJO.
Control preexisting diarrhea before starting VONJO treatment. Manage diarrhea with antidiarrheal medications, fluid replacement, and dose modification. Upon initiation of therapy, prescribe an antidiarrheal medication (eg, loperamide) and instruct the patient to treat diarrhea promptly at the first onset of symptoms (change in frequency or consistency of bowel movements) after starting VONJO. Interrupt or reduce VONJO dose in patients with significant diarrhea despite optimal supportive care.
Thrombocytopenia: VONJO can cause worsening thrombocytopenia. VONJO dosing was reduced due to worsening thrombocytopenia in 2% of patients with preexisting moderate to severe thrombocytopenia (platelet count <100 x 109/L). VONJO dosing was reduced due to worsening thrombocytopenia in 2% of patients with preexisting severe thrombocytopenia (platelet count <50 x 109/L).
Monitor platelet count prior to VONJO treatment and as clinically indicated during treatment. Interrupt VONJO in patients with clinically significant worsening of thrombocytopenia that lasts for more than 7 days. Restart VONJO at 50% of the last given dose once the toxicity has resolved. If toxicity recurs, hold VONJO. Restart VONJO at 50% of the last given dose once the toxicity has resolved.
Prolonged QT Interval: VONJO can cause prolongation of the QTc interval. QTc prolongation of >500 msec was higher in VONJO-treated patients than in patients in the control arm (1.4% vs 1%). QTc increase from baseline by 60 msec or higher was greater in VONJO-treated patients than in control arm patients (1.9% vs 1%). Adverse reactions of QTc prolongation were reported for 3.8% of VONJO-treated patients and 2% of control arm patients. No cases of torsades de pointes were reported.
Avoid use of VONJO in patients with a baseline QTc of >480 msec. Avoid use of drugs with significant potential for QTc prolongation in combination with VONJO. Correct hypokalemia prior to and during VONJO treatment. Manage QTc prolongation using VONJO interruption and electrolyte management.
Major Adverse Cardiac Events (MACE): Another Janus associated kinase (JAK)-inhibitor has increased the risk of MACE, including cardiovascular death, myocardial infarction, and stroke (compared to those treated with TNF blockers) in patients with rheumatoid arthritis, a condition for which VONJO is not indicated.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with VONJO particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur.
Thrombosis: Another JAK-inhibitor has increased the risk of thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis (compared to those treated with TNF blockers) in patients with rheumatoid arthritis, a condition for which VONJO is not indicated.
Patients with symptoms of thrombosis should be promptly evaluated and treated appropriately.
Secondary Malignancies: Another JAK-inhibitor has increased the risk of lymphoma and other malignancies excluding non-melanoma skin cancer (NMSC) (compared to those treated with TNF blockers) in patients with rheumatoid arthritis, a condition for which VONJO is not indicated. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with VONJO, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy, and patients who are current or past smokers.
Risk of Infection: Another JAK-inhibitor increased the risk of serious infections (compared to best available therapy) in patients with myeloproliferative neoplasms. Serious bacterial, mycobacterial, fungal, and viral infections may occur in patients treated with VONJO. Delay starting therapy with VONJO until active serious infections have resolved.
Observe patients receiving VONJO for signs and symptoms of infection and manage promptly. Use active surveillance and prophylactic antibiotics according to clinical guidelines.
- Interactions With CYP3A4 Inhibitors or Inducers: Coadministration of VONJO with strong CYP3A4 inhibitors or inducers is contraindicated. Monitor for increased adverse reactions of VONJO when administered with moderate CYP3A4 inhibitors.
Adverse Reactions
The most frequent serious adverse reactions occurring in ≥3% patients receiving VONJO 200 mg twice daily were anemia (8%), thrombocytopenia (6%), pneumonia (6%), cardiac failure (4%), disease progression (3%), pyrexia (3%), and squamous cell carcinoma of skin (3%).
Fatal adverse reactions among patients treated with VONJO 200 mg twice daily included events of disease progression (3%), and multiorgan failure, cerebral hemorrhage, meningorrhagia, and acute myeloid leukemia in <1% of patients, respectively.
The most common adverse reactions (reported in ≥20% of patients) include diarrhea, thrombocytopenia, nausea, anemia, and peripheral edema.
Specific Populations
Pregnancy: Advise pregnant women of the potential risk to a fetus. Consider the benefits and risks of VONJO for the mother and possible risks to the fetus when prescribing VONJO to a pregnant woman.
Lactation: It is not known whether VONJO is excreted in human milk. Because of the potential for serious adverse reactions in the breastfed child, advise patients that breastfeeding is not recommended during treatment with VONJO, and for 2 weeks after the last dose.
Infertility: Pacritinib reduced male mating and fertility indices in BALB/c mice. Pacritinib may impair male fertility in humans.
Hepatic Impairment: The recommended starting dose of VONJO in patients with severe hepatic impairment [Child-Pugh C] is 100 mg twice daily.
Renal Impairment: Avoid use of VONJO in patients with eGFR <30 mL/min.
Please see the full Prescribing Information for VONJO.
For statutory pricing disclosures, visit sobi.com/usa/en/state-disclosure-requirements.